In the world of pharmaceutical litigation, the acronym “MDL” – Multidistrict Litigation – is an all-too-common part of litigators’ and clients’ daily vocabulary. This article touches briefly on what an MDL is and how one is created, then turns to its main purpose: offering a bird’s-eye view of the current (yet constantly evolving) universe of pharmaceutical MDLs.
BASIC ROADMAP OF MDL PRACTICE
An MDL is a creature of federal statute. Authorized by 28 U.S.C. §1407, any party – defendant or plaintiff – may request an MDL in any federal district court in the country. Not to be confused with a class action, the practical effect of a new MDL means that a single federal district court is vested with the authority to manage any number of individual cases that have been deemed similar enough to be handled on a collective basis, at least through the discovery process.
The statute itself contains only two threshold criteria: first, that there are civil actions pending in different districts, and second, that the cases involve “one or more common questions of fact.”1 The mechanics of requesting an MDL begin with filing a Motion to Transfer with the governing authority, the Judicial Panel on Multidistrict Litigation (“JPML” or “the Panel”).2 Based in Washington, D.C., with its own Clerk and staff, the JPML consists of seven federal judges, no two of whom may be from the same circuit.3 Members are appointed to serve on the JPML by the Chief Justice of the United States Supreme Court.4 The Panel is chaired today by Judge Sarah S. Vance (U.S. District Court, Eastern District of Louisiana), who assumed this role in August 2014 following the long tenure of Judge John Heyburn II. Three current members – Judges Ellen Segal Huvelle, Catherine Perry and R. David Proctor – are new additions to the Panel as of 2013-2014.5
The Panel meets roughly every two months in different locations. At these scheduled sessions, the JPML hears argument on Motions to Transfer (i.e., to create an MDL) and also rules on (usually without oral argument) other MDL-specific filings.6 As pertinent here, if the Panel (a) concludes that the civil actions in question present one or more common questions of fact, and (b) further determines that transfer to a single district court for pretrial proceedings “will be for the convenience of parties and witnesses and will promote the just and efficient conduct of such actions,” the motion will be granted.7
The logistics of transferring cases from various districts to the newly minted MDL court (i.e., the “transferee court”) is beyond the scope of this article, as is the process for remanding and/or trying cases in the MDL. Instead, this article provides a snapshot of how pharmaceutical MDLs stack up in the grand scheme of MDLs nationwide, and further provides detail on current pharmaceutical MDLs in terms of who (courts, judges), what (products, numbers of actions and plaintiffs) and where (locations of MDL courts).
THE LANDSCAPE OF NATIONWIDE MDLS
An MDL can be created for almost any imaginable civil action. Common MDL subject matter includes antitrust, intellectual property, securities, products liability, contract litigation, sales practices and employment, as well as a catchall “miscellaneous” category that runs the gamut of topics. For those in the trenches of pharmaceutical litigation, it may be initially surprising to learn that pharmaceutical MDLs do not dominate other types of MDLs – at least in terms of the overall number of MDLs nationwide. Those same folks in the trenches, however, will not be surprised to hear that pharmaceutical MDLs overwhelmingly lead in terms of the number of active cases per MDL category.
OVERALL MDL NUMBERS
Statistics compiled for this article, obtained from the JPML’s October 15, 2014 official reports, identify a nationwide total of 281 MDLs considered “open” or current. The JPML categorizes pending MDLs via 10 categories of actions or “docket types,” currently identified as follows:
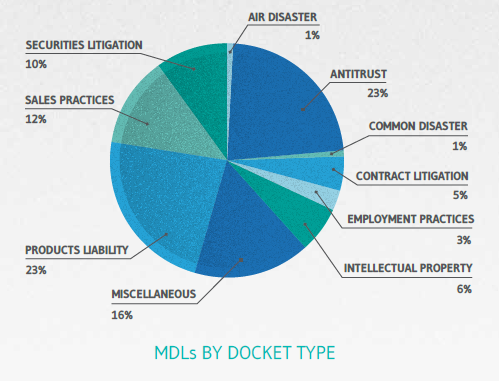
As the chart above shows, Products Liability and Antitrust are roughly equal in terms of the total number of active MDLs (65 and 64, respectively). Pharmaceutical MDLs, however, far outpace Antitrust MDLs in terms of active member actions (cases) – with pharma at nearly 116,000 cases and antitrust at approximately 1,600.
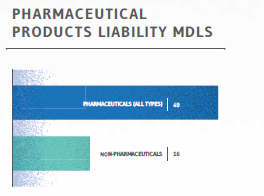
PHARMACEUTICAL VS. NON-PHARMA PRODUCTS LIABILITY MDLS
Further analysis of the 65 current MDLs making up the Products Liability category reveals that 49 MDLs involve some type of pharmaceutical product:
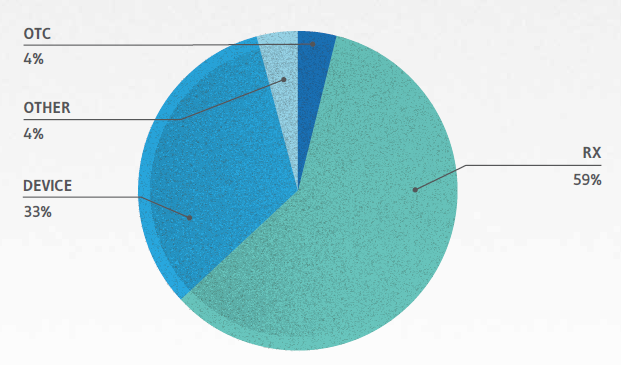
Of the 49 pharmaceutical MDLs, more than one-half (59%) involve prescription drug products. Another 33% involve medical devices, and a small fraction (4%) involve over-the-counter products:
PHARMACEUTICAL PRODUCTS LIABILITY MDLS BY PRODUCT
PHARMACEUTICAL MDLS: WHO, WHAT, WHEN, WHERE, HOW LONG
According to JPML reporting, currently active pharmaceutical MDLs include the following 49 matters, with case captions substantially and intentionally abbreviated here. Note that several of these MDLs are, for practical purposes, closed – or are close to it – yet for consistency and writing purposes, statistics were obtained and compiled from JPML reporting as of October 15, 2014. The list is organized by year of creation of the MDL; it shows that a handful of old MDLs are still on the books, and without question, new requests for MDLs continue to be granted – with more than a dozen pharmaceutical MDLs having been created over the past two years and still active. These statistics further reveal that pharmaceutical MDLs currently account for 116,000 active member actions (cases) today:
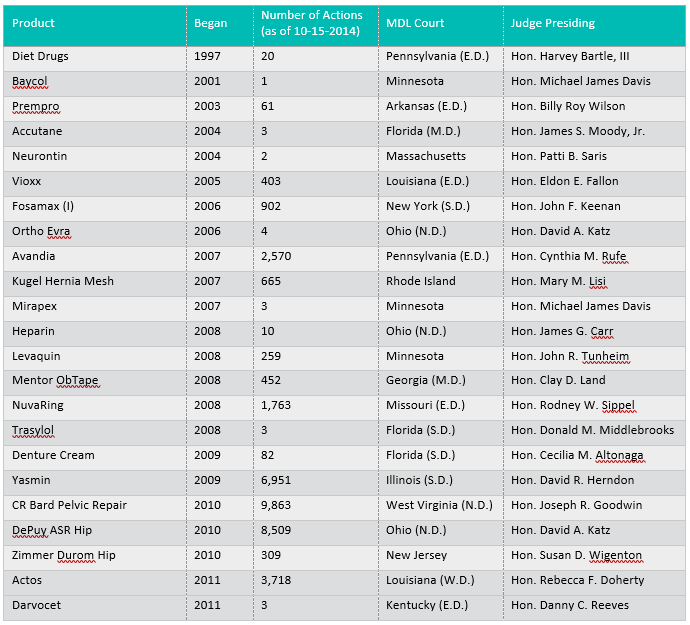
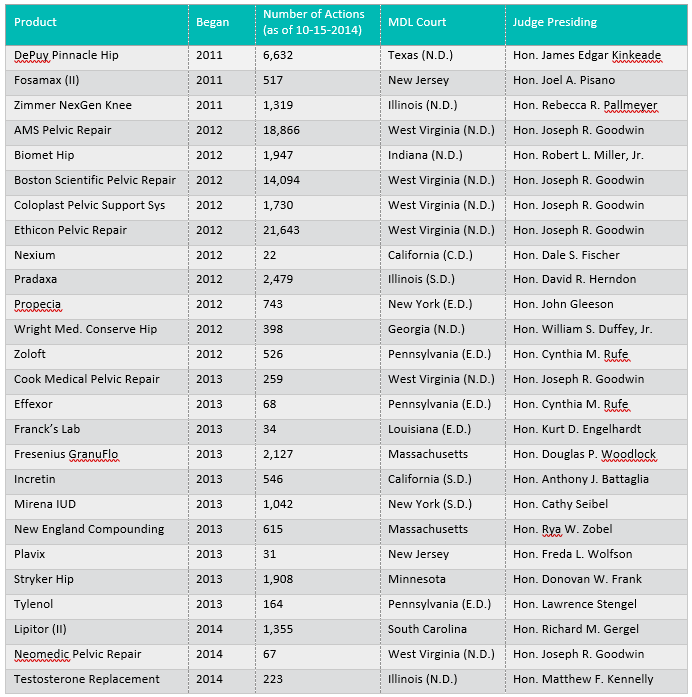
PHARMACEUTICAL MDLS: WHERE
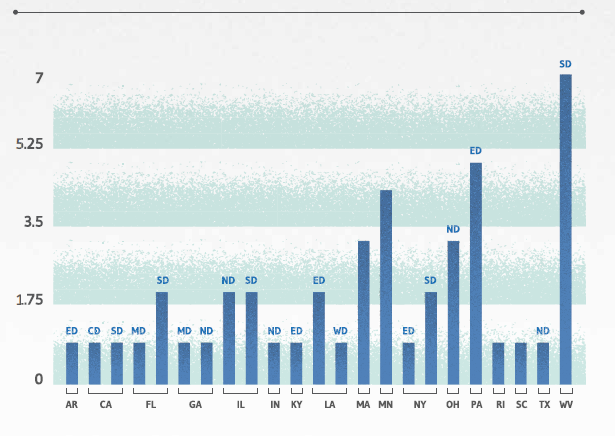
Pharmaceutical MDLs are housed across the country, with the vast majority – all except three – found in courts in the eastern half of the U.S., as reflected in the chart below and graphic on the following page. Note, however, that reporting the locations of pharmaceutical MDLs must be taken with the proverbial grain of salt: consider that Judge Joseph R. Goodwin (Northern District of West Virginia) is alone presiding over seven MDLs involving pelvic mesh products by different manufacturers. The pelvic mesh MDLs, collectively, are reported to total more than 66,500 member actions – thus representing 57% of all pharmaceutical MDL plaintiffs nationwide.
PHARMACEUTICAL MDLS BY LOCATION
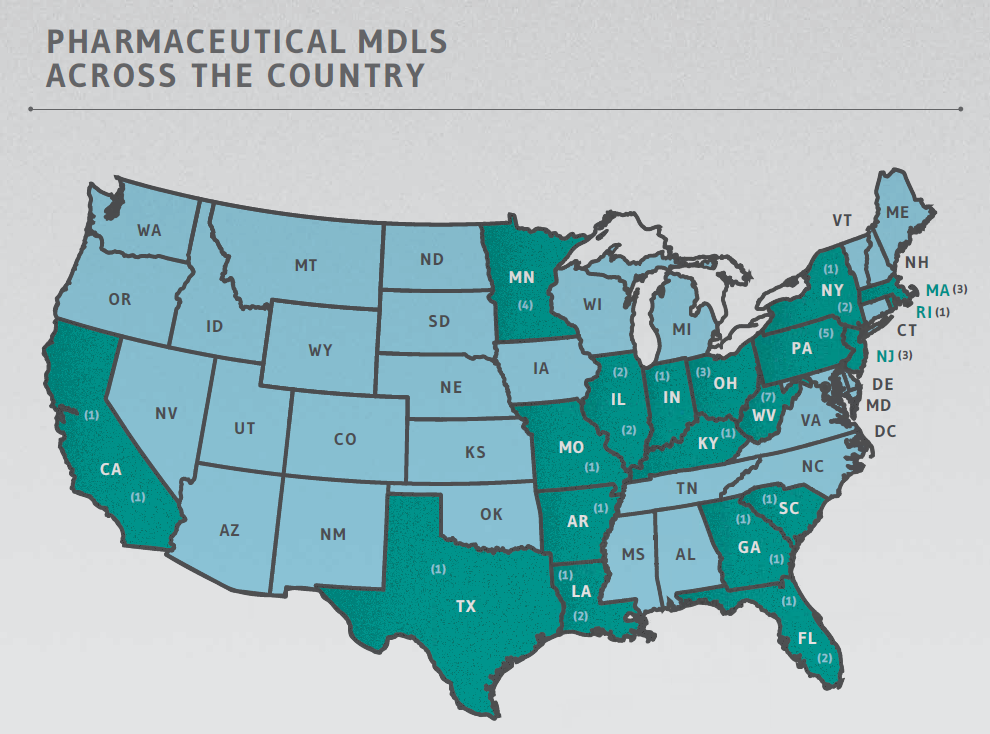
CONCLUSION
Given the vagaries of overall civil litigation at
any point in time, an across-the-board comparison of nationwide MDLs is not feasible
nor readable outside a treatise on the topic. What is evident, however, is that
pharmaceutical MDLs play a significant role in the nationwide MDL scene, in
terms of types, numbers, lengths of time and locations of the MDL courts
overseeing them.
[1] 28 U.S.C. § 1407(a).
[2] 28 U.S.C. § 1407(a).
[3] 28 U.S.C. 1407(d).
[4] 28 U.S.C. § 1407(d).
[5] Roster of Current and Former Judges of the United States Judicial Panel on Multidistrict Litigation, http://www.jpml.uscourts.gov/sites/jpml/files/Panel%20Judges%20Roster-10-16-2014_0.pdf (last accessed Oct. 17, 2014).
[6] A list of upcoming hearings may be found at http://www.jpml.uscourts.gov/hearing-information (last accessed Oct. 17, 2014).
[7] 28 U.S.C. § 1407(a)-(c).
Finis
Citations
- 28 U.S.C. § 1407(a). Jump back to footnote 1 in the text
- 28 U.S.C. § 1407(a). Jump back to footnote 2 in the text
- 28 U.S.C. 1407(d). Jump back to footnote 3 in the text
- 28 U.S.C. § 1407(d). Jump back to footnote 4 in the text
- Roster of Current and Former Judges of the United States Judicial Panel on Multidistrict Litigation, http://www.jpml.uscourts.gov/sites/jpml/files/Panel%20Judges%20Roster-10-16-2014_0.pdf (last accessed Oct. 17, 2014). Jump back to footnote 5 in the text
- A list of upcoming hearings may be found at http://www.jpml.uscourts.gov/hearing-information (last accessed Oct. 17, 2014). Jump back to footnote 6 in the text
- 28 U.S.C. § 1407(a)-(c). Jump back to footnote 7 in the text